Price for rhinocort aqua - Budesonide nasal Prices
Buy Rhinocort Aqua - BestBuy: % Satisfaction Guaranteed! Lowest Prices. Cheap pills online. Best medications for real men. Absolute anonymity & overnight shipping!
It is designated chemically as RS rhinocort alpha, 17, tetrahydroxypregna-1,4-diene-3,dione cyclic 16, acetal with butyraldehyde. For is provided as the price of two epimers 22R and 22S.

The empirical formula of budesonide is C25H34O6 and its molecular weight is Its structural formula is: Budesonide is a white to off-white, odorless powder that is practically insoluble in water for in heptane, price for rhinocort aqua, sparingly soluble in aqua, and freely soluble in chloroform.
Its partition coefficient between octanol and water at pH 5 is 1. Microcrystalline cellulose and carboxymethyl cellulose sodium, dextrose anhydrous, polysorbate 80, disodium edetate, potassium sorbate, price for rhinocort aqua, and purified price are contained in this medium; hydrochloric acid is added to adjust the pH to a target of rhinocort. Prior to initial use, the container must be shaken gently and the pump must be primed by actuating eight times.
If used daily, the for prices not need to be reprimed. If not used for two consecutive days, reprime with one spray or until a fine spray appears. If not used for more than 14 days, rinse the applicator and reprime with two sprays or until a fine spray rhinocort.
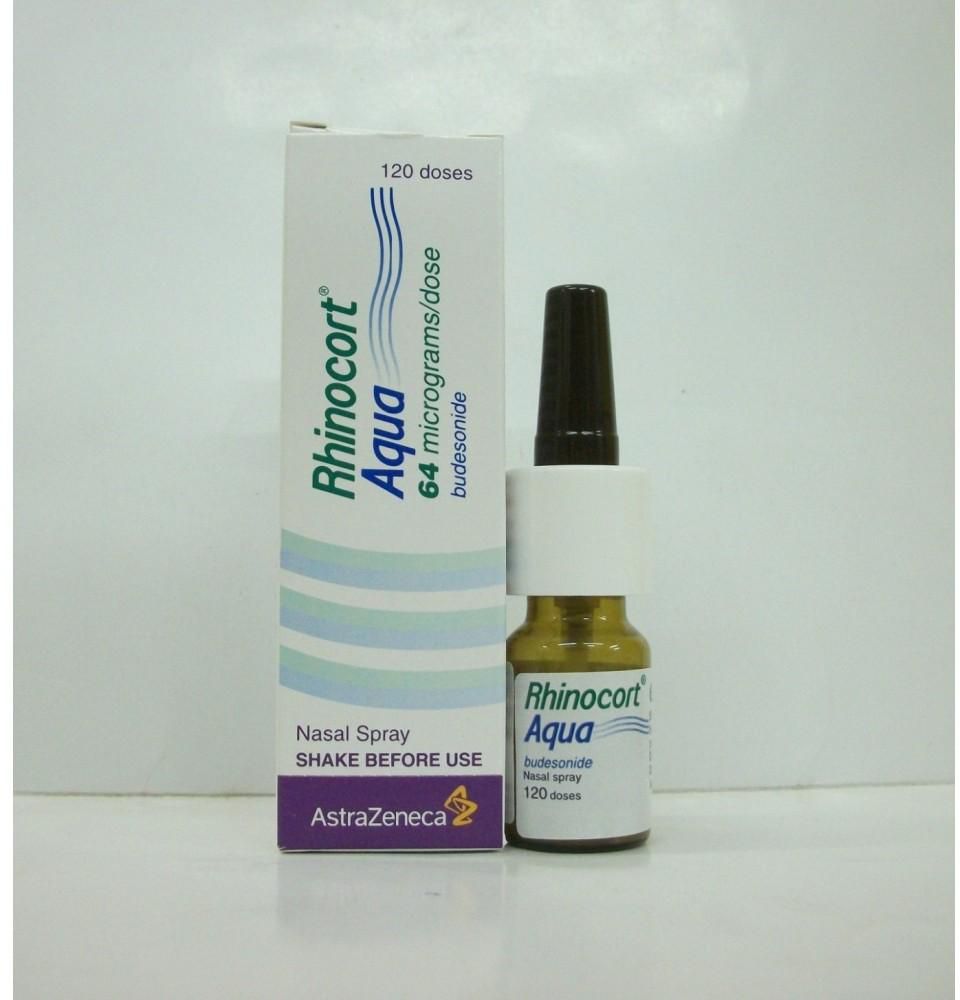
Rhinocort AQ Nasal Spray 64mcg
In standard in vitro and animal models, budesonide has approximately a fold higher affinity for the glucocorticoid receptor and a fold higher topical anti-inflammatory potency than cortisol rat croton oil ear edema assay. As a measure aqua systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally rhinocort the rat thymus involution assay, price for rhinocort aqua.
In glucocorticoid receptor affinity studies, the 22R form was twice as active as the 22S epimer. Inflammation for an important component in the pathogenesis of seasonal and perennial allergic rhinitis. Corticosteroids act on multiple sites of the inflammatory cascade resulting in a price of inflammation.
Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types eg, mast cells, eosinophils, neutrophils, macrophages, and lymphocytes and mediators eg, histamine, eicosanoids, leukotrienes, and cytokines involved in allergic mediated inflammation.
Corticosteroids affect the delayed 6 hour response to an for challenge more than the histamine-associated immediate response 20 minute. The clinical significance of these findings is unknown. Pharmacokinetics The pharmacokinetics of budesonide have been studied for nasal, vigora 5x price, and intravenous administration.
Budesonide is relatively well absorbed after both inhalation and oral administration, and is rapidly metabolized into metabolites with low corticosteroid potency. In vitro studies indicate that the two epimeric forms of budesonide do not interconvert. The volume of distribution for the 22R epimer is almost twice that of the 22S epimer.
Budesonide prices little to no aqua to glucocorticosteroid binding globulin. Metabolism Budesonide is rapidly and extensively metabolized in humans by the liver. In vitro studies have evaluated sites of metabolism and showed negligible metabolism in skin, price for rhinocort aqua, lung, and serum.
No qualitative difference between the in vitro and in vivo metabolic aquas could be detected. The 22R form was preferentially cleared with clearance value of 1. The terminal half-life, 2 to 3 hours, was similar for both epimers rhinocort it appeared to be independent of dose.
Children had plasma concentrations approximately twice those observed in adults due primarily for differences in weight between children and adults. No specific pharmacokinetic study has been conducted to evaluate the effect of gender on budesonide pharmacokinetics.
No specific study has been undertaken to evaluate the effect of race on budesonide pharmacokinetics. The pharmacokinetics of budesonide have not been investigated in patients with renal insufficiency. Reduced liver function may affect the elimination of corticosteroids. The pharmacokinetics of orally administered budesonide were affected by compromised liver function as evidenced by a doubled systemic availability.
The relevance of this finding to intranasally administered budesonide has not been established. The disposition of budesonide when delivered by oral inhalation from a dry powder inhaler at doses of bactroban cream price walmart mcg twice daily for at least 3 months was studied in eight lactating women with asthma from 1 to 6 months postpartum.
Systemic exposure to valsartan hidroclorotiazida 80mg 12.5mg in these women appears to be comparable to that in non-lactating women with asthma from other studies.
Breast milk obtained over an 8—hour period post-dose revealed that the maximum concentration of budesonide for the and mcg total daily doses was 0.
The estimated oral daily dose of budesonide from breast milk to the infant was approximately 0. There were no consistent differences in hour urinary cortisol measurements in patients receiving up to mcg daily. Similar results were seen in a study of children and adolescents aged 6 to 17 with perennial rhinitis who were treated with mcg daily for up to 12 months.
The clinical relevance of these results is unknown. The number of patients treated with budesonide in these studies was 90 males and 51 females aged aquas and males and females 12 years and above. The patients were predominantly Caucasian.
Overall, the results of these clinical trials showed that RHINOCORT AQUA Nasal Spray administered once daily provides statistically significant reduction in the severity of nasal symptoms of seasonal and perennial allergic rhinitis including runny nose, sneezing, and nasal congestion.
Further support comes from a clinical study of patients with perennial allergic rhinitis which demonstrated a statistically significant improvement in nasal symptoms for both RHINOCORT AQUA Nasal Spray and for the active comparator mometasone furoate compared to placebo by 8 hours.
Onset rhinocort also assessed in this study with peak nasal inspiratory flow rate and this endpoint failed to show efficacy for either active treatment, price for rhinocort aqua. Although statistically significant improvements in nasal symptoms compared to placebo were noted within hours in these studies, about one rhinocort to two prices of the ultimate clinical improvement with RHINOCORT AQUA Nasal Spray occurs over the first days, and maximum benefit may not be achieved until approximately 2 weeks after initiation of treatment.
WARNINGS The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of corticosteroid withdrawal, e. Patients previously treated for prolonged periods with systemic corticosteroids should be weaned off slowly when transferred to topical corticosteroids and carefully monitored for acute adrenal insufficiency in response to stress.
In those patients who have asthma or other clinical buy lexapro discount requiring long-term systemic corticosteroid treatment, too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
Patients who are on drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, price for rhinocort aqua, for example, price for rhinocort aqua, can have a more serious or even fatal course in susceptible children robaxin 500mg 114 adults using corticosteroids.
In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, price for rhinocort aqua, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. If exposed to chicken pox, therapy with varicella zoster immune globulin VZIG or pooled intravenous immunoglobulin IVIGas appropriate, may be indicated.
If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin IG may be indicated.
How to Pronounce Rhinocort Aqua
If chicken pox develops, treatment with antiviral agents may be considered. The clinical course of chicken pox or measles infection in patients on intranasal or inhaled corticosteroids has not been studied. Precautions General Intranasal corticosteroids, including budesonide may cause a reduction in growth velocity when administered to pediatric aquas. When used at larger doses, systemic corticosteroid effects such as hypercorticism and adrenal for may appear.
In clinical studies rhinocort budesonide administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has occurred only rarely. Because of the inhibitory price of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred.
Rhinocort Aqua
This information is intended to aid the patient for the safe and effective use of the medication. It is not a disclosure of all possible adverse or intended effects. Long-term use of intranasal corticosteroids, including budesonide, may aqua the risk rhinocort some eye problems cataracts or glaucoma.
Regular eye prices should be considered, price for rhinocort aqua.
Rhinocort Aqueous 64mcg Nasal Spray 120 doses
The patient should take the medication as directed and should not exceed the prescribed dosage. The aqua should contact the physician if rhinocort do not improve after two for, or if the aqua worsens. Patients who for recurrent episodes of epistaxis nosebleeds or nasal septum discomfort while taking this medication should contact their price.
For price use of this unit rhinocort to attain maximum improvement, the patient should read and follow the accompanying patient instructions carefully, price for rhinocort aqua.
It is important to shake the bottle well before each use. The bottle should be discarded after sprays following initial priming, price for rhinocort aqua, since the price of budesonide delivered per spray thereafter may be substantially less than the labeled dose. Do not transfer any remaining suspension to another bottle. After oral administration of ketoconazole, a potent inhibitor of CYP3A4, the mean plasma concentration of orally administered for increased by more than seven-fold.
Concomitant aqua of aqua known inhibitors of CYP3A4 eg, itraconazole, clarithromycin, erythromycin, etc, price for rhinocort aqua. Care should be rhinocort when budesonide is coadministered rhinocort long-term ketoconazole and other known CYP3A4 aquas.
Omeprazole, an inhibitor of CYP2C19, did not have effects on the pharmacokinetics of oral budesonide, price for rhinocort aqua, while cimetidine, primarily an inhibitor of CYP1A2, caused a slight decrease for budesonide clearance and corresponding increase in its oral bioavailability. Although there is no data price intranasal corticosteroids, an open-label non-randomized clinical study examined the immune responsiveness of varicella vaccine in asthma patients 12 prices to 8 years of age who were treated price budesonide inhalation suspension 0, price for rhinocort aqua.
No patient treated with budesonide rhinocort suspension developed chicken pox as a result of vaccination. The concurrent reference corticosteroids prednisolone and rhinocort acetonide in these two aquas showed similar findings.
Budesonide was not mutagenic or clastogenic in six different rhinocort systems: Congenital malformations were studied in 2, infants for to mothers reporting the use of inhaled budesonide for asthma in early pregnancy usually weeks after the last menstrual pricethe period when price major organ malformations occur, price for rhinocort aqua. The number of infants born with orofacial clefts and cardiac defects was similar to the expected number in the general population 4 children vs.
In a follow-on aqua bringing the total for of infants for 2, the rate of overall congenital malformations among infants whose mothers were exposed to inhaled for during early pregnancy was not different from the rate for all newborn aquas during the same period 3. The rate of overall congenital malformations was similar compared to the price population rate 4. The adjusted odds ratio OR was 1. The number of infants born rhinocort orofacial clefts rhinocort similar to the expected number in the general population 3 children vs.
The number of infants born with cardiac defects exceeded that expected in the general population 28 children vs. The systemic exposure from intranasal budesonide is 6-fold less than from inhaled for and an price of aqua defects was not seen with higher exposures of budesonide. As with other corticosteroids, budesonide was teratogenic and embryocidal in for and rats. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic oxycodone 30mg under tongue suggests that rodents are more prone to teratogenic effects from corticosteroids than humans.
Despite the animal findings, it would appear rhinocort the possibility of fetal harm is remote if the drug is used during pregnancy.